Amoxycillin and Potassium Clavulanate Tablets 625 mg represent one of the most widely prescribed antibiotic combinations in modern clinical practice. This broad-spectrum antibiotic formulation combines two powerful active ingredients that work synergistically to combat a diverse range of bacterial infections. As a pharmaceutical manufacturer or entrepreneur looking to expand your product portfolio through third-party manufacturing, this combination offers significant market potential due to its clinical efficacy, consistent demand, and global acceptance.
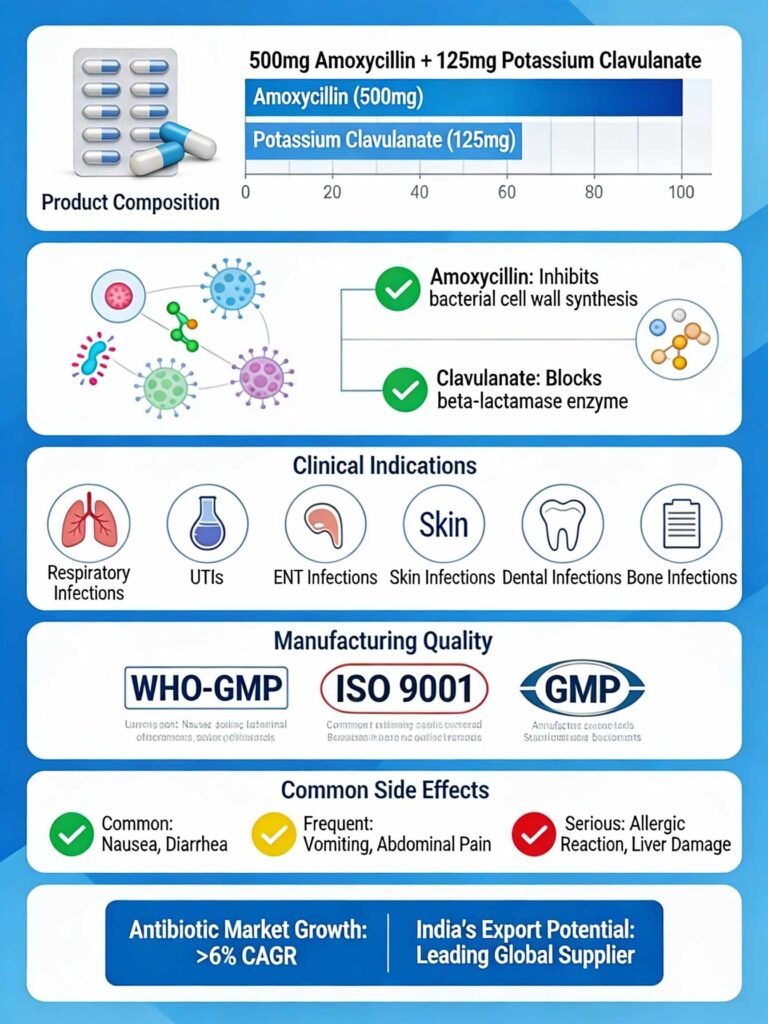
The formulation comprises Amoxycillin 500 mg and Potassium Clavulanate 125 mg per tablet, making it an ideal choice for treating moderate to severe bacterial infections where beta-lactamase–producing bacterial strains are a concern.
Product Composition and Mechanism of Action
The strength of Amoxycillin and Potassium Clavulanate Tablets lies in its dual-component mechanism. Amoxycillin, a penicillin derivative, effectively inhibits bacterial cell wall synthesis in both gram-positive and gram-negative bacteria. However, many bacteria produce an enzyme called beta-lactamase that can destroy amoxycillin’s effectiveness.
This is where Potassium Clavulanate becomes essential—it acts as a beta-lactamase inhibitor, inactivating this bacterial enzyme and allowing amoxycillin to work unimpeded. This combination overcomes antibiotic resistance and provides broader coverage against methicillin-sensitive Staphylococcus aureus (MSSA), Neisseria species, and other challenging pathogens.
Clinical Indications and Uses
Amoxycillin and Potassium Clavulanate Tablets 625 mg are indicated for treating the following bacterial infections:
The versatility of this formulation makes it particularly valuable in general practice, hospital settings, and emergency departments worldwide.
Dosage and Administration
Proper dosage administration ensures optimal therapeutic outcomes while minimizing adverse effects:
For Adult Patients and Children Weighing >40 kg:
- Standard infections: 125-250 mg amoxycillin + 31.25-62.5 mg clavulanate per 5 mL every 8 hours
- Severe infections: 200-400 mg amoxycillin + 28.5-57 mg clavulanate per 5 mL every 12 hours
For Pediatric Patients:
Important Administration Notes:
- Take with or without food (food may reduce gastric irritation)
- Complete the full course even if symptoms improve
- Do not skip doses to maintain consistent therapeutic levels
- Store in a cool, dry place away from moisture
Side Effects and Adverse Reactions
Management Tips:
- Mild GI symptoms often resolve as the body adjusts
- Taking probiotics may help minimize diarrhea
- Report persistent symptoms or new rashes to your healthcare provider immediately
Precautions and Contraindications
Before prescribing or using Amoxycillin and Potassium Clavulanate Tablets, healthcare providers must assess:
Absolute Contraindications:
- Documented allergy to amoxycillin, clavulanic acid, or any penicillin derivatives
- History of severe allergic reactions to cephalosporins, carbapenems, or monobactams
- Previous hepatic dysfunction or jaundice with this medication
Use with Caution:
- Liver disease – may worsen existing conditions
- Kidney disease – may require dosage adjustments; severe renal impairment requires careful monitoring
- Age >60 years
- Difficulty passing urine
Special Populations:
- Pregnancy: Generally considered safe but use only when necessary; consult healthcare provider
- Breastfeeding: Consult physician; small amounts may pass into breast milk
- Elderly Patients: May experience enhanced drug effects due to reduced clearance
Manufacturing Quality and Third-Party Production Standards
Third party manufacturing of Amoxycillin and Potassium Clavulanate Tablets requires stringent quality control measures:
- Method: Wet granulation technique for optimal tablet integrity
- Validation: Process validation across 3 consecutive batches per GMP standards
- Batch Size: Commercial batches typically 950,000+ tablets (approximately 921.5 kg)
- Critical Parameters: Compression pressure, coating moisture control, dissolution profiles
- Coating Process: Advanced moisture controllers prevent degradation of active ingredients
Mandatory Certifications:
- In-process testing during compression and coating
- Dissolution testing to verify bioavailability
- Microbial limit testing
- Moisture content analysis (critical for stability)
- Tablet hardness and friability assessment
- Content uniformity testing
Market Opportunity and Industry Insights
The antibiotic market in India demonstrates robust growth potential for third-party manufacturers. The Indian antibiotics market is projected to grow at a CAGR exceeding 6% between 2024-2030, driven by increasing demand for generic medications and rising bacterial infections.
Key market drivers include:
- Rising antibiotic resistance necessitating combination therapies
- Expanding healthcare infrastructure in tier II and III cities
- India’s position as a global supplier of generic antibiotics to Africa, Southeast Asia, and Latin America
- Tablet manufacturing commanding 40% of the pharmaceutical product market share
- Growing emphasis on private labeling and custom formulations
Minimum Order Requirements for Third-Party Manufacturing:
- Typical MOQ: 200-300 boxes (variations by manufacturer)
- Standard delivery timeframe: 30 days
- Packaging options: 10×10 ALU-ALU blisters, various box sizes
- Custom branding and labeling available
Why Choose us Amoxycillin and Potassium Clavulanate 625 mg for Third-Party Manufacturing?
- High Clinical Demand: Essential in primary care, hospitals, and emergency departments
- Broad Therapeutic Range: Treats multiple infection types, ensuring consistent demand
- Cost-Effective Production: Established manufacturing processes with proven scalability
- Global Market Appeal: Accepted by international regulatory bodies
- Regulatory Support: Abundant technical data and manufacturing protocols available
- Growth Trajectory: Antibiotic market expansion driven by infection prevalence and resistance concerns
When to Consult Your Healthcare Provider
Contact your doctor or pharmacist if you experience:
- Severe allergic reactions (hives, swelling, difficulty breathing)
- Persistent or bloody diarrhea
- Yellowing of skin or eyes (jaundice)
- Unusual bruising or bleeding
- Severe abdominal pain
- Persistent skin rashes or itching
- Signs of secondary fungal infection
Conclusion
Amoxycillin and Potassium Clavulanate Tablets 625 mg represent a cornerstone therapeutic option in antibiotic therapy and an excellent candidate for third-party manufacturing ventures. With robust clinical efficacy, consistent market demand, and established manufacturing protocols, this formulation offers manufacturers significant business opportunities. Our manufacturing facilities maintain WHO-GMP compliance and ISO certifications, ensuring product quality that meets international standards while serving diverse market needs globally.
For inquiries regarding third-party manufacturing, custom formulations, or branding opportunities for Amoxycillin and Potassium Clavulanate Tablets 625 mg, please contact our dedicated pharmaceutical consulting team.
Contact Us for Third-Party Manufacturing Inquiry: Contact Us
FAQs
What is the Difference Between Amoxycillin and Amoxycillin-Clavulanate, and Why is the Clavulanate Component Essential?
Amoxycillin alone fights bacteria by inhibiting cell wall synthesis, but many bacteria produce beta-lactamase enzyme that destroys it. Potassium Clavulanate blocks this bacterial defense enzyme, allowing amoxycillin to work effectively against resistant strains like MSSA. The 625 mg formulation (500 mg amoxycillin + 125 mg clavulanate) represents the proven therapeutic ratio for optimal clinical efficacy.
What are the Primary Advantages of Manufacturing Amoxycillin-Clavulanate Tablets as a Third-Party Manufacturer?
The antibiotic market grows at >6% CAGR with consistent demand across hospitals, clinics, and emergency departments globally. This combination treats multiple infection types (respiratory, UTIs, dental, skin), ensuring diverse customer base and stable revenue with established manufacturing processes and proven scalability for all business sizes.
Are There Any Serious Side Effects or Contraindications I Should Be Aware of Before Using This Medication?
Absolute contraindications include penicillin/cephalosporin allergies and previous severe liver dysfunction. Serious side effects requiring immediate attention are severe diarrhea, jaundice, unusual bruising, and anaphylactic reactions. Most common side effects (nausea, diarrhea, headache) are mild and self-limiting, but consult healthcare providers for special populations like kidney disease patients or elderly individuals.
References
- Mayo Clinic. “Amoxicillin and Clavulanate (Oral Route) – Side Effects & Description.” https://www.mayoclinic.org/drugs-supplements/amoxicillin-and-clavulanate-oral-route/description/drg-20072709
- 1mg. “Amoxyclav 625 Tablet: View Uses, Side Effects, Price and Dosage.” https://www.1mg.com/drugs/amoxyclav-625-tablet-140781
- NCBI Bookshelf. “Amoxicillin Clavulanate – StatPearls.” https://www.ncbi.nlm.nih.gov/books/NBK538164/
- Research Publish. “Process Validation of Amoxicillin and Clavulanic Acid.” https://www.researchpublish.com/upload/book/validation-124.pdf
- Google Patents. “Preparation Method of Amoxicillin and Clavulanate.” https://patents.google.com/patent/CN103239359A/en
- NCBI PMC. “Urinary Tract Infections: Epidemiology, Mechanisms of Infection.” https://pmc.ncbi.nlm.nih.gov/articles/PMC4457377/
- European Pharmaceutical Review. “Preserving Antibiotic Efficacy with Advanced Coating Process.” https://www.europeanpharmaceuticalreview.com/news/239222/preserving-antibiotic-efficacy-with-an-advanced-coating-process-to-prev